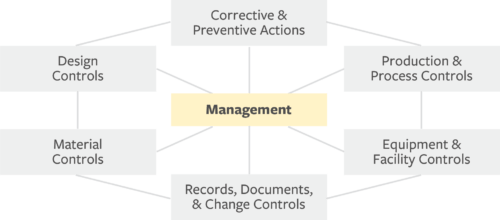
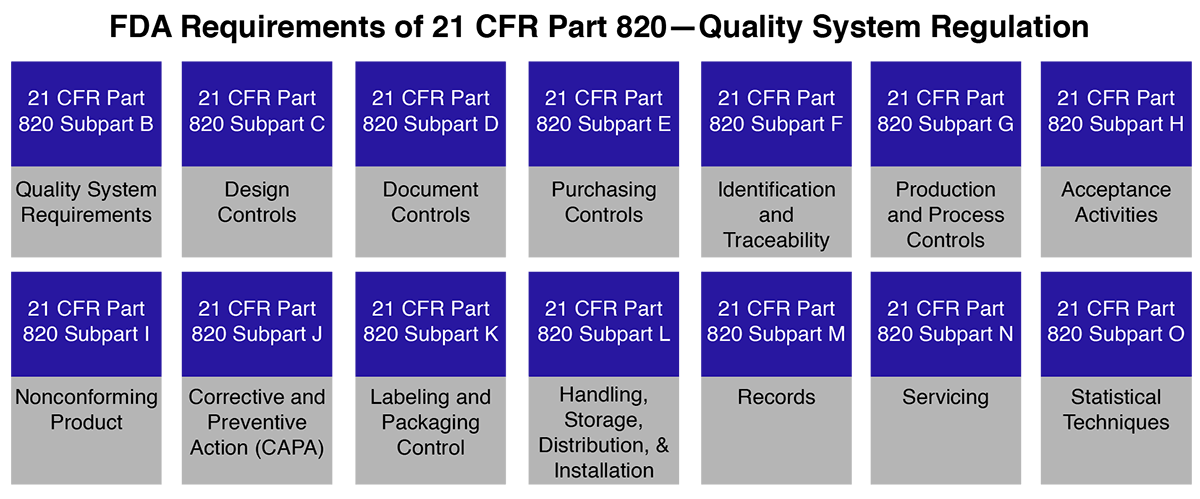
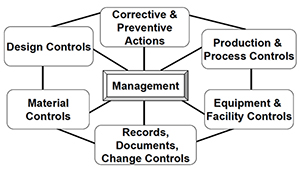
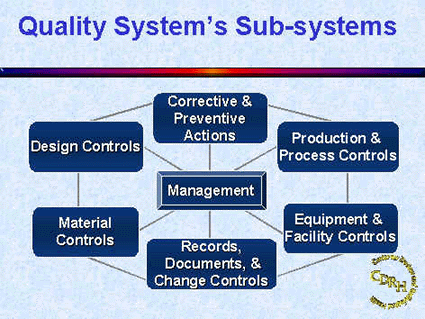
Amazon.com: FDA Quality System Regulation for Medical Devices (21 CFR Part 820): A Practitioner's Guide to Management Controls eBook : Daugherty, D: Kindle Store

Operon Strategist - FDA 21 CFR Part 820 Quality System Regulation Consultant Services for Medical Device Manufacturers. US FDA QSR, GMP Guidelines, FDA (510k), CE Mark Regulatory. https://operonstrategist.com/services/turnkey-project/quality-management ...

FDA Issues Quality Management System Regulation: Final Rule Amending the Quality System Regulation - ICE









%20Ultimate%20Guide%20to%2021%20CFR%20Part%20820%20%E2%80%94%20FDA%20Quality%20System%20Regulation%20(QSR)%20for%20Medical%20Devices.png?width=250&height=324&name=(cover)%20Ultimate%20Guide%20to%2021%20CFR%20Part%20820%20%E2%80%94%20FDA%20Quality%20System%20Regulation%20(QSR)%20for%20Medical%20Devices.png)